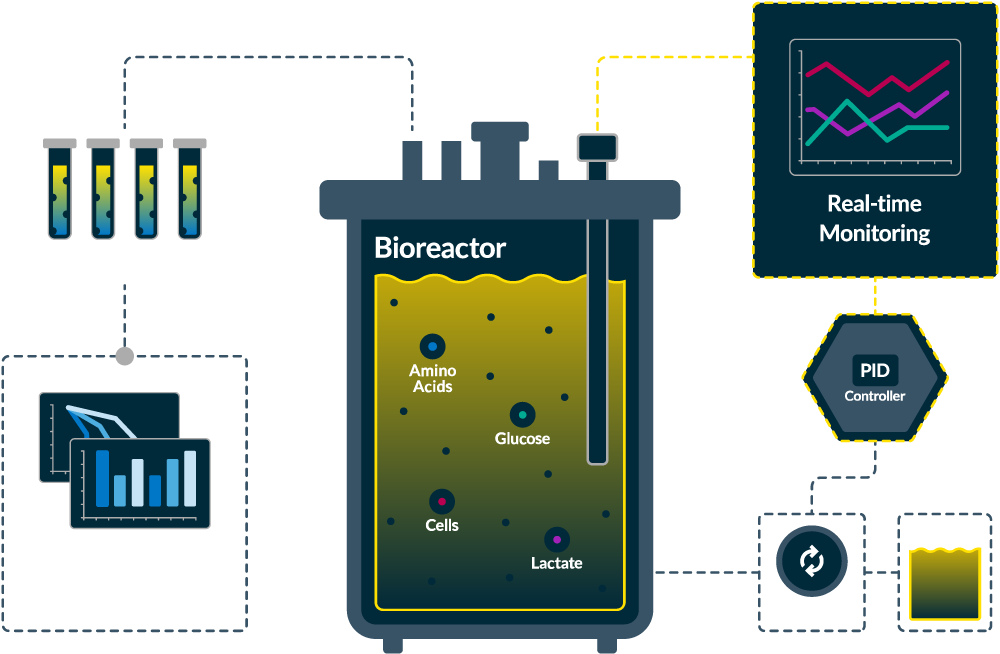
Cell Therapy Process Analytics
Don’t let variability compromise your cell therapy process. Our advanced analytical solutions empower you to monitor critical quality attributes in real-time, ensuring consistent cell expansion and product quality. By integrating real-time analytics, you can make informed decisions swiftly, reduce process development timelines, and enhance scalability.
Overcoming Manufacturing Challenges in Cell Therapy
Cell therapy is increasingly becoming a viable therapeutic alternative to traditional treatments for a wide range of diseases. However, the complexities of manufacturing process characterization and control severely limit their availability and wide adoption. Dealing with the variability of starting materials, meeting strict manufacturing timeframes, and maintaining compliance with Current Good Manufacturing Practices (cGMP) make it difficult to develop and implement a robust manufacturing process. Transitioning away from manual manufacturing protocols and towards adaptive process strategies requires careful monitoring and control of critical process parameters (CPPs) with the help of at-line, on-line, and in-line Process Analytical Technologies (PAT).
 At-line Monitoring of Amino Acids
At-line Monitoring of Amino Acids
Amino acids are critical to cellular function and viability, but different cells may consume them at different rates. Monitoring concentrations of amino acids during cell expansion helps prevent their depletion that may cause a batch failure.
 Real-time Glucose Monitoring
Real-time Glucose Monitoring
Glucose is the primary energy source and its concentration in the media regulates metabolic pathways and is linked to proliferation, metabolites production, and phenotype expression. Continuous glucose measurement can be used for control of substrate feeding or media exchange pumps.
 Continuous Lactate Monitoring
Continuous Lactate Monitoring
Excessive accumulation of lactate can inhibit cell growth and decrease cytokine secretion. It can also be used to estimate cell count, predict cell expansion rate, determine optimal harvest time.
 In-line Biomass Measurement
In-line Biomass Measurement
In-situ Total Cell Density (TCD) measurement can be used to ensure that appropriate cell densities are achieved, especially in adherent cell cultures where cell counting is difficult to perform.

Cell Therapy Media is Critical
Cell therapy that utilizes primary and stem cells introduce distinct challenges in the selection, development, and optimization of media. The degree of cell expansion, differentiation, and functional activation achieved ex vivo can differ markedly across patients and between donors. Given that the formulation of cell culture media directly influences critical cell characteristics—including growth rates, health, and functional capabilities—it’s imperative to comprehensively assess how different media components affect the Critical Quality Attributes (CQAs). These attributes play a pivotal role in determining the therapeutic effectiveness of cell therapy.
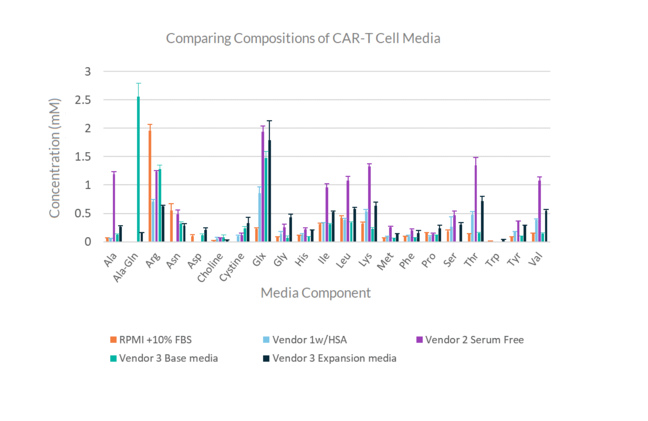
Media Fingerprinting & Screening
Screen and compare commercially available cell therapy media and ensure consistency of media formulations from run to run. With REBEL XT, you get at-line media analysis of 30+ components in under 10 minutes.
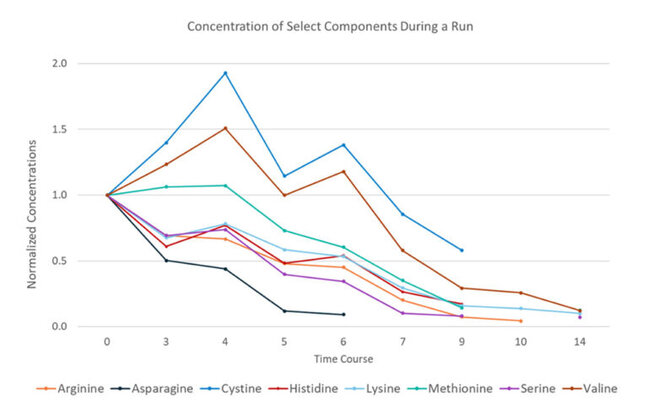
Accelerate Media Optimization
Developing and optimizing media formulation necessitates careful monitoring of key nutrients’ consumption, metabolite production and their collective effects on process efficiency and product quality attributes. Waiting weeks for cell culture media analysis results is not feasible. REBEL XT provides a solution with its rapid analysis turnaround time, small sample volumes, and minimal sample prep.
Media Composition Can Make a Big Impact on Your Process
Analyzing Serum-Free Media for Cell Therapy Applications
Design an ideally formulated, chemically defined serum-free media.

Comparative Media Analysis in Cell Therapy Development
Understand the impact of media components on critical quality attributes of cell therapy products.
Spent Media Analysis to Improve Cell Therapy Manufacturing
Profiling T Cell metabolic requirements throughout the manufacturing process with spent media analysis.
Fingerprinting Mesenchymal Stem Cells (MSCs) Media
Evaluate impact of supplements on overall MSC media composition.
Ensure Process Robustness with Real-Time Monitoring
A well-characterized and controlled production process is crucial for effectiveness of cell therapy and necessitates integration of Process Analytical Technology (PAT) solutions with automated cell therapy manufacturing systems. MAVEN and MAVERICK easily integrate with cell therapy manufacturing systems and deliver continuous monitoring and control of critical process parameters (CPPs) such as glucose, lactate and total biomass, enhancing process understanding, ensuring robustness and reducing human intervention. With REBEL XT amino acids concentrations in the media can be frequently monitored during cell expansion processes to ensure that any variations in cell growth and health were not due to unknown changes in expected media formulation.

Timely Process Insights in Cell Therapy Manufacturing
Our suite of devices provides at-line, on-line or in-line monitoring for cell expansion process.
Implement Process Analytical Technology (PAT) in Your Cell Therapy Workflows
Historically, automated cell therapy manufacturing systems had limited real-time monitoring capabilities due to lack of robust and compatible process analytical technologies. MAVEN and MAVERICK are offered with a variety of single-use and reusable process interfaces and multiple analog and digital communication options making integration possible with a number of manufacturing automation systems.

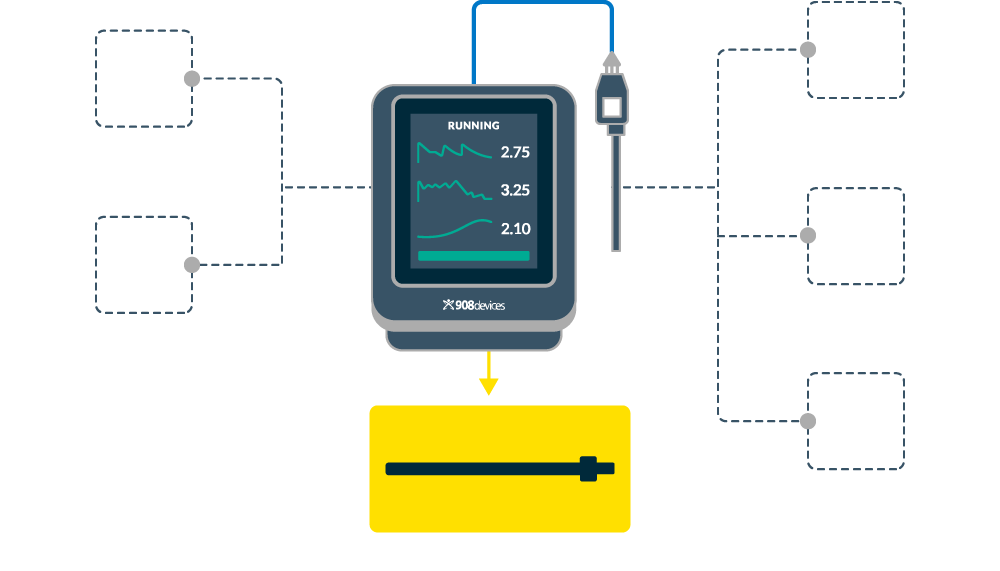

REBEL XT At-line Media Analyzer
At-line Analysis for Cell Culture Media
Built on the proven foundation of the award winning REBEL analyzer, the REBEL XT System continues to challenge the limits of traditional lab testing—delivering the next level of at-line cell culture media analysis with greater reliability, streamlined usability, and tighter analytical performance. Designed for real-world cell culture media process development, it delivers faster, real-time insights into media composition—giving you the data you need, exactly where and when you need it.

MAVERICK In-line Critical Process Parameter Monitoring
Process Analytical Technology for In-line Bioprocess Analysis
This easy-to-use process analytical technology device offers in-line bioprocess analysis and control with no complex modeling. Measure multiple critical parameters across media, across processes, across mammalian cell lines, across scales.

MAVEN Online Glucose and Lactate Monitoring
Take command of your cell culture process with the MAVEN® Online Glucose and Lactate Monitoring System. Delivering continuous, sample-free glucose data every 2 minutes, MAVEN automates feeding strategies, reduces variability, and frees your team from manual sampling. Built to integrate seamlessly, it brings precision and efficiency to every run—without disrupting your workflow.
Cell Therapy Collaboration Announcements

908 Devices and Terumo BCT
908 Devices and Terumo Blood and Cell Technologies collaborate to add on-line analytics to Quantum Flex cell therapy expansion systems.

908 Devices and Cellares
908 Devices and Cellares are collaborating to integrate our MAVERICK in-line analyzer into Cellares’ Cell Shuttle, a fully integrated cell therapy manufacturing platform.