AVIPure® AAV Affinity Resins
AVIPure® AAV Affinity Resins provide capture step purification of adeno-associated virus (AAV) 2, 5, 8, and 9 vectors with potential alkaline clean-in-place regeneration for improved process economics. A 50 µm cross-linked agarose matrix bead ensures compatibility with standard bioprocess columns and flow rates.
High performance at a lower price point
AAV process cost reduction
- ~20% lower resin cost/L
- > 20 cycle re-use, NaOH stable
- High binding capacity, yield, purity
Pre-packed or loose resin
- Available now: AAV2, AAV5, AAV8, AAV9
- Available pre-packed in OPUS® Columns
Secure supply
- Redundant manufacturing at 3+ sites
- Worlds largest ligand manufacturer at 4+ sites
- 10+ year supply agreements
 HIGH PERFORMance
HIGH PERFORMance
High performance at a lower price point
AVIPure® AAV 2, 5, 8, and 9 Affinity Resins meet or exceed the performance metrics of resins developed on alternative platforms with the additional benefit of robust alkaline stability. Affinity resins offer the ability to simplify downstream processing. NaOH stable affinity resins simplify processes and dramatically improve overall process economics.
- Maintain high host cell protein (HCP) clearance and residual DNA clearance levels after 20 cycles
- Across life of the resin using 0.1 to 0.5 M NaOH for routine cleaning and sanitization
- High dynamic binding capacity even at short residence time enables use with pre-concentrated feed or direct capture:
- >2 x 1014 vp/mLresin at 1 minute residence time
- >7 x 1014 vp/mLresin at 4 minute residence time
Impact of Low pH Affinity Chromatography Elution on AAV Transduction
Affinity chromatography enables effective AAV purification for gene therapy, but the use of acidic pH (pH 2–3) for elution raises concerns about virus stability. Studies across different pH conditions and temperatures showed minimal impact on infectivity at 25°C, with some aggregation and loss of infectivity for AAV8 at 40°C. Throughout downstream purification, virus aggregation was linked to capsid concentration rather than the capture process, and immediate pH neutralization after elution helps mitigate stability risks.
Retain performance with NaOH CIP regeneration
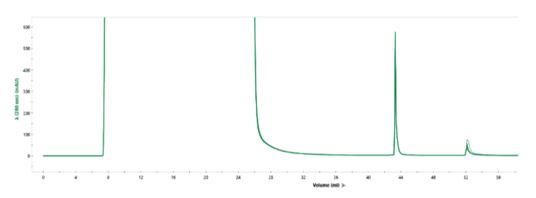
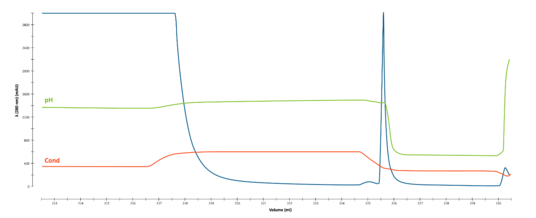
Overlay of every fifth cycle from a single AVIPure® AAV2 column used in a 31-cycle study demonstrating robust chromatographic performance after cleaning in place with NaOH. Total NaOH exposure time at final cycle was 15 hours.
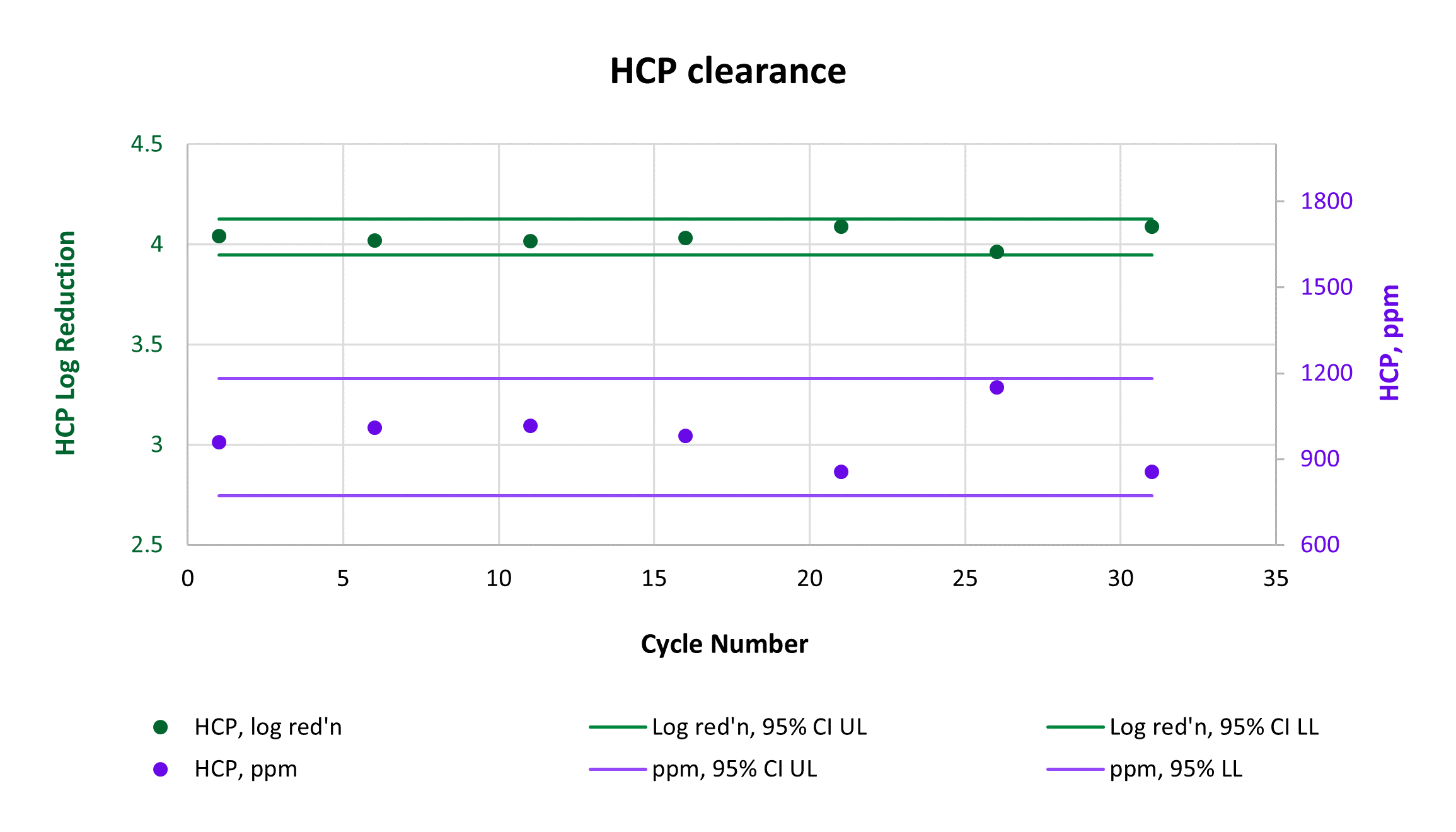
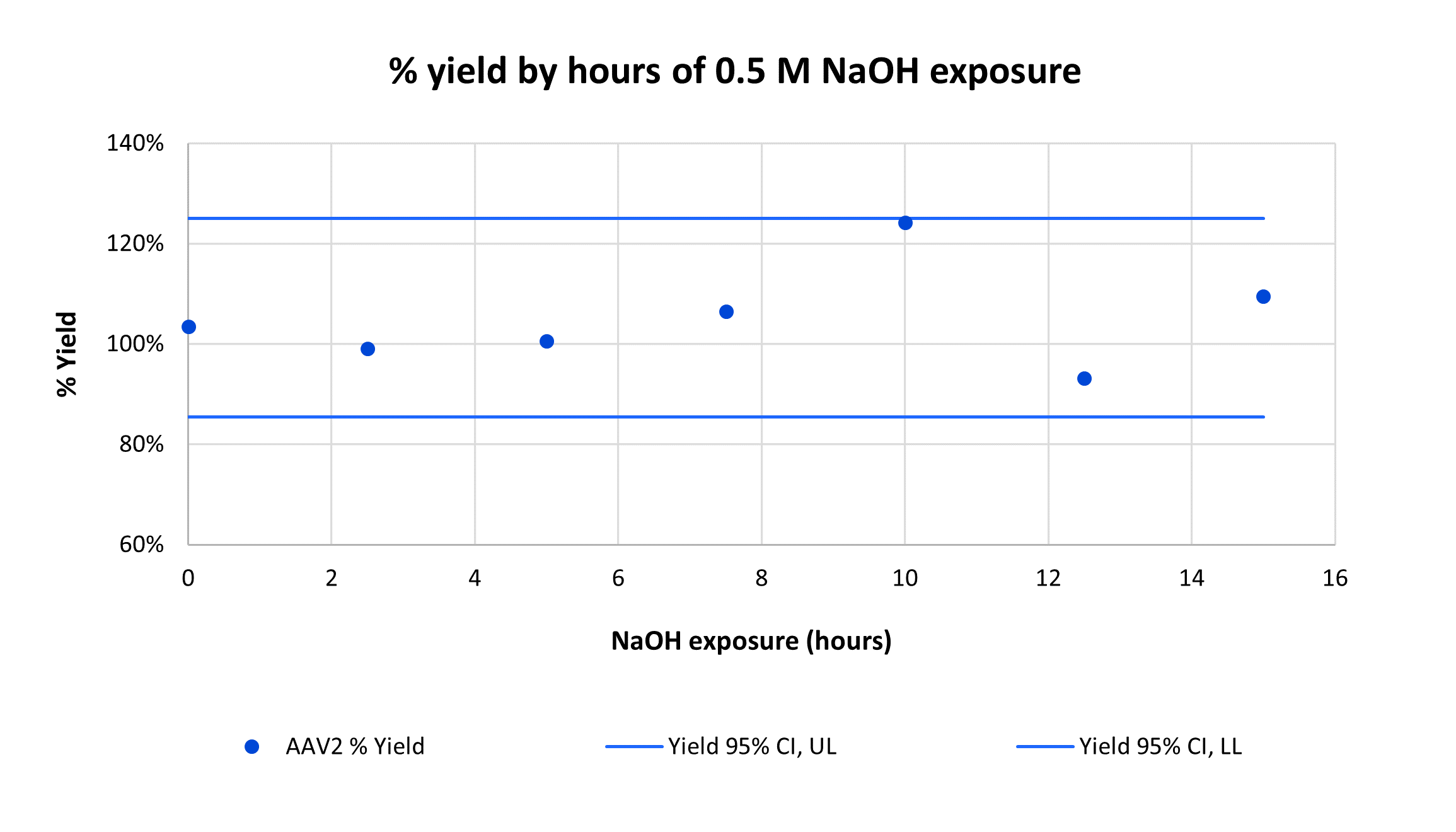
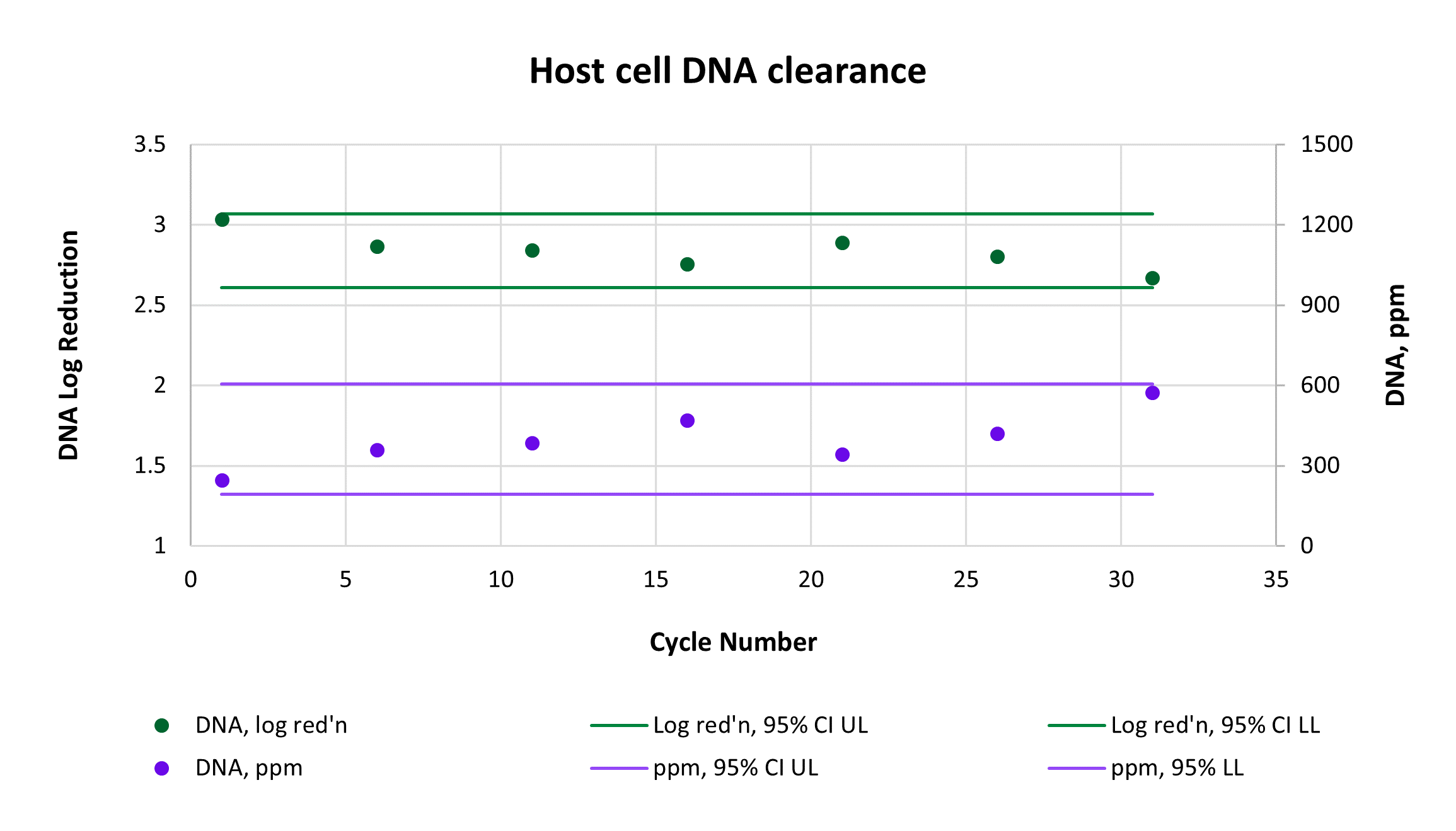
Consistently high yield and clearance of host cell protein (HCP) and host cell DNA (hcDNA) were achieved across 31 cycles on a single AVIPure® AAV2 column. Yield was measured using commercially available AAV2 Progen ELISA kits, HCP was measured by HCP ELISA, and hcDNA was measured by Picogreen assay. Average yield was 105%, average 4.0 log reduction of HCP to 977 ppm, and average 2.8 log reduction of hcDNA to 400 ppm.
Bind with 2-minute residence time, Clear more HCP and DNA
Bind, wash and elute direct capture of AAV cell culture feed (not concentrated) stream using AVIPure® AAV2 Resin.
A 2 -minute residence time enabled elimination of an existing pre-concentration step. Product was eluted in 50 mM glycine, 150 mM NaCl, 0.01% P188, pH 3 with 85% recovery.
AVIPure® AAV2 Resin and a competing AAV affinity resin were run under identical conditions. The eluted product was analyzed for purity by SDS-PAGE, HCP and residual DNA.
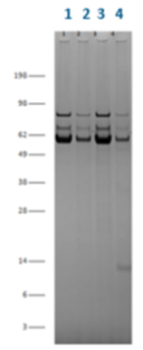
AVIPure AAV2 Resin and a competing AAV affinity resin were run under identical conditions (2 minute residence time) with the eluted product analyzed for purity by SDS-PAGE, HCP and residual DNA. AVIPure AAV2 Resin delivered purified product with 7-fold lower residual HCP, 1.5X lower residual DNA and fewer impurity proteins by SDS-PAGE.
Results
AVIPure® AAV2 Resin delivered purified product with 7-fold lower residual HCP.
AVIPure® AAV2 Resin delivered purified product with 1.5X lower residual DNA and fewer impurity proteins by SDS-PAGE.
 SPEED TO MARKET
SPEED TO MARKET
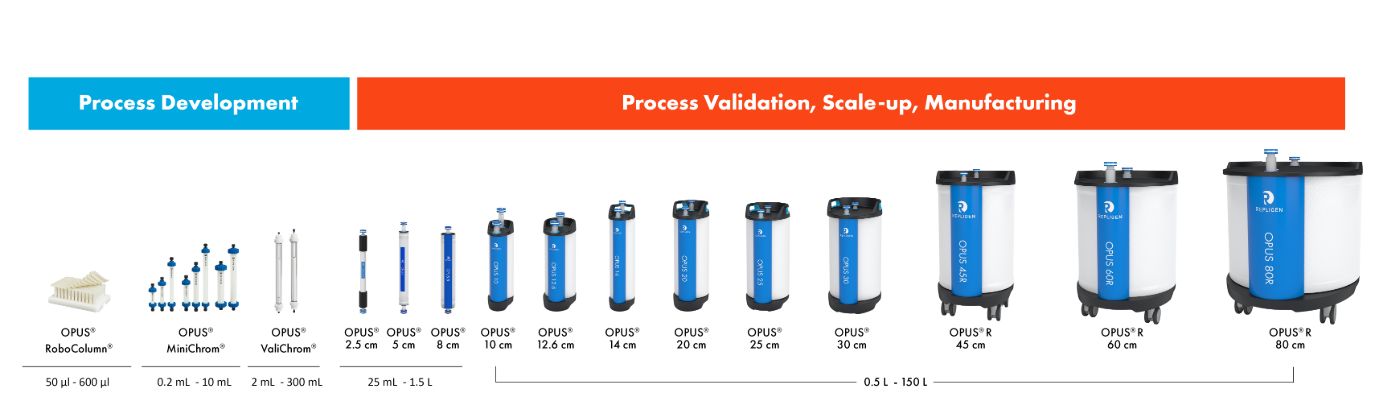
Available pre-packed in OPUS® Columns for quick deployment
AVIPure® AAV affinity resins are available in pre-packed and pre-qualified OPUS® Columns for rapid GMP implementation as well as in loose resin formats.
OPUS® Columns allow you to progress from development to manufacturing scale in weeks, using pre-packed and pre-qualified chromatography columns.
AVIPure® AAV2, 5, 8, 9 Resin characteristics
|
Category |
Description |
|---|---|
| Base Matrix | Cross-linked agarose, spherical |
| Particle size (d50V) | ~ 50 µm |
| Ligand |
AAV2, AAV5, AAV8: Alkali-tolerant recombinant protein (animal free) AAV9: Alkali-tolerant, peptide (synthetic) |
| Coupling chemistry |
AAV2: Thiol |
| Binding capacity |
High dynamic binding capacity even at short residence time enables use with pre-concentrated feed or direct capture: |
| Buffer compatibility | Stable to all commonly used aqueous buffers, including 8 M urea, 6 M guanidine hydrochloride, ethylene glycol, and detergents |
| Solvent compatibility | Water, alcohol (0–20% v/v), acetonitrile, 1–2 M acetic acid, other common organic solvents |
| pH stability | 1-13 |
| Cleaning-in-place stability | 0.1-0.5 M NaOH |
| Pressure/flowa | 3 bar at >300 cm/hr |
| Maximum pressure (ΔP)a | 40 psi |
| Temperature stability | 2–40 °C |
| Delivery conditions | 2% benzyl alcohol |
| Storage | 2–8 °C, 2% benzyl alcohol or 18-20% ethanol; do not freeze |
| a In a 2.6 x 20 cm column pressure packed at 4 bar | |
Manufacturing Centers of Excellence
Repligen develops and manufactures products for the biopharmaceutical industry under an ISO 9001 quality management system. We focus on the timely delivery of high quality, consistent and robust products, to ensure business continuity for our customers.
Repligen manufacturing sites are located in Massachusetts, California, and New Jersey in the United States and in Sweden, France, The Netherlands, Germany and Estonia.


Customer First.
Support is part of the Repligen DNA. Our goal is to provide exceptional customer experience, and to support the efficient and successful adoption and implementation of all Repligen products and services.
- Field Application Support
- Customer Service
- Field Service Engineers





