
At BioProcess International 2024 in Boston, see firsthand how our latest innovations for bioprocessing can help you intensify your process and optimize productivity and efficiency.
Attend Panel Discussions and Talks
- Learn about innovative solutions to Improve production and manufacturing for emerging advanced therapies.
- Understand how to navigate manufacturing complexities and regulatory challenges while leveraging CDMO expertise in cell and gene therapy.
- Gain insights into the current state of the bioprocessing industry, including funding trends, major M&A impacts, and macroeconomic factors.
View Featured Products
Visit Booth #116 to view the Featured Products and to talk to our applications experts about every step of your bioprocess.
View Posters
Take a sneak peek at the two new posters that will be presented at BPI Boston 2024 and review the other posters presented at past BPI meetings.
Gene Therapy and Viral Vector Manufacturing
Viral vector manufacturing processes suffer from low productivity and reproducibility. Integrating an advanced manufacturing platform can enhance viral vector yield, which reduces cost of producing AAV and lentivirus.
At Repligen, we understand these challenges and have optimized solutions to address them.
A highlight of our gene therapy solutions:
-
Integrate tangential flow depth filtration (TFDF) system in upstream processing into the production bioreactor makes the process more scalable
-
Increase efficiency in downstream processing with the RS TFF System and KRM Chromatography System
-
Get quick and direct total viral vector analysis during development with the FlowVPX System technology with variable pathlength technology (VPT)
Technical Panel Discussion and Presentations
Learn how to accelerate your speed to the market using innovative products to manufacture gene therapies, mAbs, and biologics.
Join us for a Panel Discussion
9/24 from 4:30 PM to 5:30 PM
Track: Development of Emerging Cell & Gene Therapies
Moderator and panelist: Dr. Rachel Legmann, Ph.D, Sr. Director of Technology, Gene Therapy at Repligen
Key topics
- Navigating the complex grey area of manufacturing for Phase 1 and 2 trials
- Keeping efficiency while complying with limited guidance
- Need conversation between regulators and developers
- Lessons learnt and case studies from companies moving between clinical phases
- Therapeutic Developer/CDMO dynamics in the phase 1 and beyond
- How to best leverage CDMO expertise and capital efficiency
Panelists:
Sarah Thomas, Sr. Vice President, Quality RENGEXBIO Inc.
Kathryn Golden, SVP, Technical Operations and Cell Manufacturing, bit.bio
Kate Rochlin, Chief Operating Officer, IN8bio
Shankar Swaminathan, PhD Team Lead, Drug Product Development, CMC-Tech Ops, Astellas Institute for Regenerative Medicine
Moderator Bio
 Rachel Legmann has more than 25 years of experience in the field of scalable biologics and gene therapy manufacturing of therapeutic products, viral vectors, and proteins for gene therapy and biologics. She completed her PhD in Food Engineering and Biotechnology at the Technion-Israel Institute of Technology, Haifa, Israel. Rachel joined Repligen, Waltham, MA, USA in 2021 as a subject matter expert, leading the global gene therapy organization and helping customers achieve their technical and operational objectives in their manufacturing of vector-based therapeutics and vaccines, with a focus on gene therapy processes including upstream, downstream, analytics, and scalability. In addition to supporting global customers and building high level networks, Rachel is supporting various internal cross-functional activities and external collaborations. Prior to joining Repligen, Rachel held several scientific and leadership roles at the Department of Microbiology and Molecular Genetics at Harvard Medical School, CRO SBH Sciences, Seahorse Biosciences (part of Agilent), CDMO Goodwin Biotechnology, and Pall Corp part of Danaher.
Rachel Legmann has more than 25 years of experience in the field of scalable biologics and gene therapy manufacturing of therapeutic products, viral vectors, and proteins for gene therapy and biologics. She completed her PhD in Food Engineering and Biotechnology at the Technion-Israel Institute of Technology, Haifa, Israel. Rachel joined Repligen, Waltham, MA, USA in 2021 as a subject matter expert, leading the global gene therapy organization and helping customers achieve their technical and operational objectives in their manufacturing of vector-based therapeutics and vaccines, with a focus on gene therapy processes including upstream, downstream, analytics, and scalability. In addition to supporting global customers and building high level networks, Rachel is supporting various internal cross-functional activities and external collaborations. Prior to joining Repligen, Rachel held several scientific and leadership roles at the Department of Microbiology and Molecular Genetics at Harvard Medical School, CRO SBH Sciences, Seahorse Biosciences (part of Agilent), CDMO Goodwin Biotechnology, and Pall Corp part of Danaher.Join us for a Breakfast Talk
9/25 from 8:15 AM to 8:45 AM
Speaker: Charles Hill, Sr. Field Application Scientist Upstream, Repligen
Abstract
The advancements of processes for monoclonal antibody (mAb) production are significantly influencing the development of emerging advanced therapies, such as cell and gene therapies. Key innovations in mAb manufacturing, across the workflow from upstream process intensification, high-capacity and mass transfer chromatography, scalable filtration technologies, and robust analytics are being adapted to meet the unique challenges of these new therapies. These processes are enhancing productivity, scalability, and consistency, which are critical for the successful commercialization of advanced therapy medicinal products (ATMPs). By leveraging the lessons learned from mAb production, the industry is creating more efficient, reliable, and scalable manufacturing platforms that are driving the next generation of life-saving therapies.
Speaker Bio

Charles Hill is a motivated upstream scientist in the biopharmaceutical industry with over seven years of experience in process intensification across various modalities. Charles is currently a senior field applications scientist at Repligen Corporation, helping companies successfully implement upstream filtration technologies like the XCell® ATF and Krosflo® TFDF®. His experience covers traditional therapeutics, gene therapy, and cell therapy applications. Charles previously worked at Bristol-Myers Squibb performing different functions in process development, characterization, and technical transfer for perfusion processes. Charles holds a bachelor's degree in biomedical engineering from Worcester Polytechnic Institute.
Join us for a Presentation
9/25 from 10:35 AM to 11:25 AM
Innovation and Community Stage (Exhibit Hall)
Speaker: Olivier Loeillot, President and CEO at Repligen
Topics
- The turnaround in the funding environment and how that is affecting the supply sector
- Major M&A over the past few months in the outsourced and supplier space
- The widening modality focus of the industry and how vendors and CDMOs are responding
- Macroeconomic factors (BIOSECURE and China, specifically)
Speaker Bio

Olivier Loeillot joined Repligen in October 2023 as President and Chief Commercial Officer (“CCO”), where he has responsibility for driving the Company’s commercial strategy and expanding the market impact of Repligen’s business units. Prior to joining Repligen, Mr. Loeillot was Chief Executive Officer (“CEO”) at Ascensus Specialties, a manufacturer of specialty chemicals for use in the life sciences and pharmaceutical markets. Mr Loeillot previously served a combined 12 years with Cytiva (a Danaher company and previously GE Healthcare Life Sciences). While at Cytiva, he served as Bioprocess President from 2018 to 2022, overseeing the overall bioprocess portfolio from cell culture media to purification resins and including process equipment, single-use technologies and enterprise solutions. Mr. Loeillot was also instrumental in building and leading the Enterprise Solutions business, managing the Bioprocess Asia business in Singapore, and directing the Genomics and Cellular Research division. Prior to Cytiva, Mr. Loeillot served a combined 12 years with Lonza, advancing to Vice President Sales, Lonza Custom Manufacturing. He also acted as Vice President of Sales for Lonza AG’s custom manufacturing business and led the Microbial Biopharmaceuticals group. Mr. Loeillot earned his M.S. in Chemistry in 1993 from the European High Institute of Chemistry of Strasbourg, France, and later completed a M.B.A. program at CESMA Business School of EM Lyon.
Featured Products
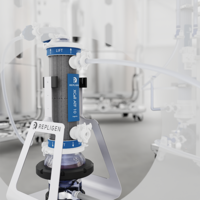
Simplify and intensify upstream bioprocessing. The XCell® ATF System, in single-use or stainless steel format, delivers high cell retention. High impact upstream solutions include N-1 perfusion, high productivity harvest, long-term perfusion and seed train intensification.

TangenX® SC TFF Device: Revolutionize tangential flow filtration processes
Eliminate the hassle and inefficiency that comes with traditional downstream filtration cassette formats. Say goodbye to cassette holders, complex setups, continued torquing, wasted time, and product loss risks. Discover TangenX® SC, the future of TFF cassette solutions for ultrafiltration and diafiltration, designed to meet the demands of today’s TFF processes while dramatically reducing setup time and minimizing product loss risk.

Game-changing TFDF® (Tangential Flow Depth Filtration) technology that simplify and intensify upstream viral vector bioprocessing, ideal for AAV, lentivirus, oncolytic, retrovirus, adenovirus, exosomes, and cells on microcarriers.

A cutting-edge single-use mixing system for bioprocess products that boasts exceptional performance for shear-sensitive substances.

Turnkey systems for tangential flow filtration (TFF), KrosFlo® Systems are compatible with single-use hollow fiber filters and/or flat sheet cassettes, modernizing how UF/DF is performed at every stage from process development to large-scale manufacturing.

At-line GMP and non-GMP testing
The CTech™ SoloVPE® System unlocks the power of Slope Spectroscopy with its unique and patented variable pathlength technology (VPT). Avoid costly dilution with this simple, yet empowering variable pathlength solution that delivers rapid and accurate results.

New for 2024
Selection of TFF Cassette Feed Screen for Efficiency
Authors: Matthew Georgiades, R&D Repligen; Daniela Soluk, Director of Process Chromatography Sales, Repligen

New for 2024
Leveraging real-time concentration data for automated UF/DF in cGMP manufacturing
Authors: Steve Cates; Ing Kathleen Mihlbachler

Advanced Technology Platform for Stem Cell-derived Exosomes Manufacturing Process
Authors: Elie Zakhem, RoosterBio; Jae Jung, RoosterBio; Cameron Garland, RoosterBio; Michael Boychyn, RoosterBio; Lauren Torres, Repligen; Mario Sinani, Repligen; Carl Breuning, Repligen; Jeremy Neidert, Repligen; Rachel Legmann, Repligen
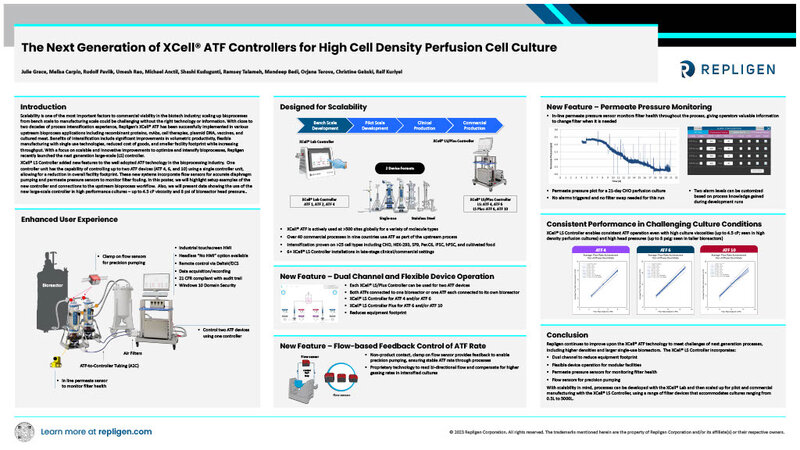
The Next Generation of XCell® ATF Controllers for High Cell Density Perfusion Cell Culture
Authors: Julie Grace, Melisa Carpio, Rudolf Pavlik, Umesh Rao, Michael Anctil, Shashi Kudugunti, Ramsey Talameh, Mandeep Bedi, Orjana Terova, Christine Gebski, Ralf Kuriyel

Disruptive Technologies Transforming Upstream Process Intensification for All Modalities
Authors: Julie Grace, Melisa Carpio, Orjana Terova, Earl Pineda

Purification Scalability and Reproducibility of AAV9 Viral Vector Moving Into Clinic
Authors: Kathleen Mihlbachler, Repligen; Corben Davis, Forge; Blake Gursky, Forge; Ganesh Krishnamoorthy, Forge; Frank Agbogbo, Forge; Rachel Legmann, Repligen

Development of an Affinity Chromatography Process for Lentiviral Vectors
Authors: Aaron Mason, Andy Politis, Kelley Kearns, Tom Scanlon, Warren Kett • Avitide, a Repligen company

Developing New Affinity Options for Albumin and Albumin Fusion Proteins
Authors: Kelley Kearns, Brandon Kier, Andy Politis, Thomas Scanlon • Avitide, a Repligen company

Solvent-free Removal of Double-stranded RNA Contaminants from mRNA
Authors: Nathaniel E Clark, Avitide, a Repligen Company

Rapid Development of Caustic Stable rAAV Affinity Resins: Novel Affinity Resins for AAV5 and AAV6 Serotypes
Authors: Laura Pickrell • Kelley Kearns • Aaron Mason • Thomas Scanlon • Avitide, a Repligen company
 02
02