Variable Pathlength Spectroscopy for Accurate Protein Concentration Measurement Using A280
Protein Concentration Measurement using A280 Overview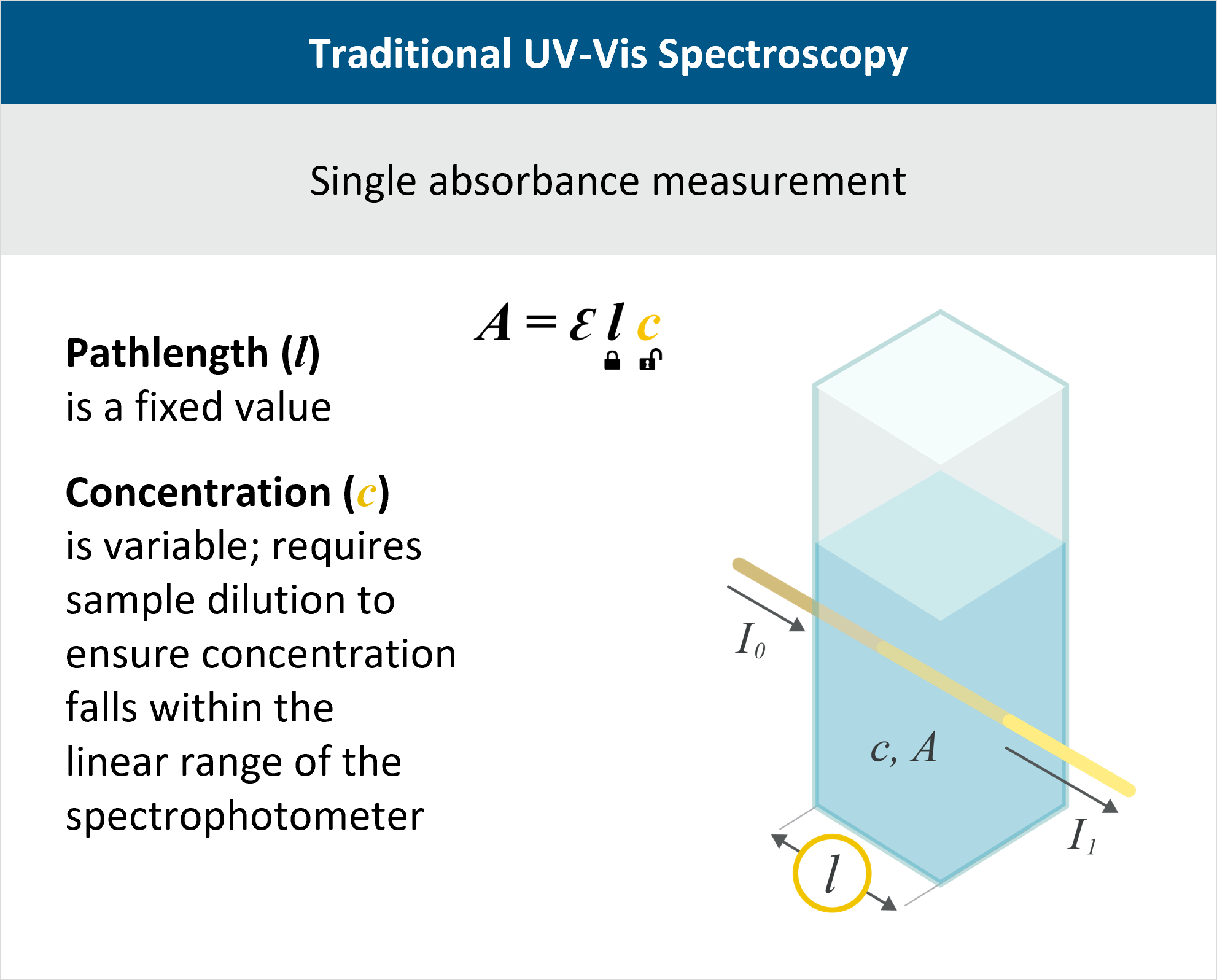
Protein concentration determination is integral to in-process control throughout biomanufacturing to monitor and optimize the production of proteins, antibodies, and other biologics. Absorbance at 280 nm, or A280, is the industry-standard method for determining protein concentration. Whether for process development, manufacturing, or quality control, accurate A280 measurements are essential for various bioprocess steps such as purification, ultrafiltration and diafiltration (UF/DF), formulation, and release testing.
A280 is an application of the Beer-Lambert law, A=εlc. If the protein’s extinction coefficient ε is known and the pathlength l is kept constant, then absorbance A is linearly proportional to concentration c.
Current Challenges
Transitioning to Continuous Processing
As bioprocessing practices transition from batch to continuous processing to enhance efficiency and productivity, there's a critical need for advanced methods for quantifying protein concentrations. Existing methods face two main challenges: measuring high protein concentrations and measuring concentration in real time.
Challenges with Traditional Spectrophotometers
New protein therapeutics, whether investigational or commercial, frequently demand high concentrations to enable effective subcutaneous administration. Traditional spectrophotometers, which use a fixed pathlength for A280 measurement, do not have a sufficient dynamic range to measure the concentration accurately at all stages of the process. Dilution techniques can bring the sample within the detectable range, but this increases the chances of variability or error due to differences in operator skill levels, preparation procedures, sample viscosity, and the need for meticulous scale calibration.
Limitations of In-line UV-Vis Sensors
In-line UV-Vis sensors provide an integrated approach for real-time monitoring of protein concentration. However, these sensors face their own challenges, notably oversaturation and limited dynamic range, especially when dealing with high-concentration biologics. The high absorbance levels of concentrated samples can surpass the sensor's detection capabilities, resulting in signal saturation and inaccurate measurements. Furthermore, the inherent limitations in dynamic range may compromise the sensor's ability to accurately capture variations in concentration across a wide spectrum, posing challenges for precise monitoring. Given the in-line nature of these sensors, dilution strategies are not viable, exacerbating the need for robust solutions to address these challenges and ensure accurate in-process monitoring of protein concentration.
The Need for Innovative Analytical Solutions
As the biopharmaceutical industry progresses towards more potent drug formulations, traditional fixed-pathlength methods struggle to meet the analytical requirements. The emergence of stronger, smaller, and more targeted batches of drugs, supporting a personalized medicine approach, underscores the need for innovative analytical solutions.
Advantages of Variable Pathlength Spectroscopy for Protein Concentration Measurement (A280)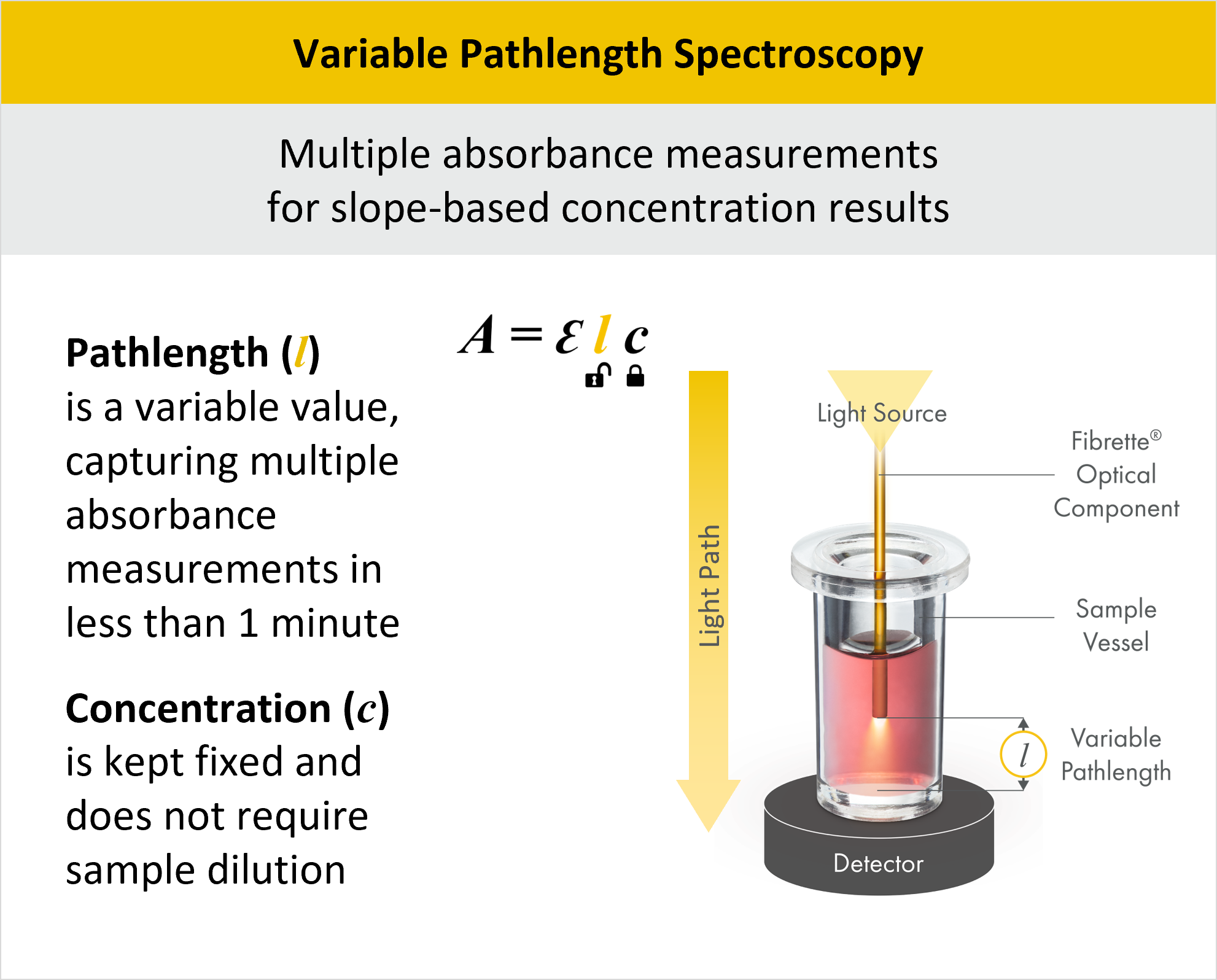
Variable Pathlength Spectroscopy operates within the framework of the Beer-Lambert Law, A=εlc. However, unlike traditional approaches where the pathlength l remains fixed, Variable Pathlength Spectroscopy strategically varies the pathlength, thereby unlocking the constraint on l. This variability in pathlength enables a wider dynamic range in concentration measurement, eliminating the need for dilutions and allowing the concentration c to remain fixed during measurement.
In this method, light enters the system through a fiber optic cable at the top of the instrument, passes through a solid Fibrette into the sample, and exits through the bottom. The pathlength is the distance between the bottom of the Fibrette and bottom of the sample vessel, which is adjusted by a stepper motor for precise control over the pathlength variation.
The CTech™ SoloVPE® and FlowVPX® Systems harness the power of Variable Pathlength Technology (VPT) to deliver rapid and precise A280 measurements for protein concentration. By utilizing VPT, our systems offer numerous advantages over traditional UV-Vis spectrophotometers. They conduct multiple absorbance measurements per sample, enabling slope-based concentration results that yield superior accuracy compared to conventional methods. This approach eliminates errors associated with additional dilutions and calculations and streamlines the measurement process. Pathlength adjustment increases the dynamic range of the system, granting the ability to measure concentrations from 0.5 to 300 mg/ml, depending on the molecule of interest, with impressive accuracy within 1%. VPT systems also include built-in quality checks to ensure reliable and consistent results across a wide concentration range.
VPT systems are available in two options for bioprocessing analytics:
CTech™ SoloVPE® System
CTech™ SoloVPE® System provides at-line concentration values in less than 2 minutes, with no need to send samples off to QC labs for process-disrupting concentration checks.
- VPT allows the direct measurement of proteins, including monoclonal antibodies (mAbs), which absorb light at 280 nm due to aromatic amino acids (primarily L-tyrosine and L-tryptophan).
- The system takes 5 to 10 absorbance data points, generating a slope regression and ensuring R2 ≥ 0.999.
- System-to-system consistency and streamlined and fast implementation and validation.
CTech™ FlowVPX® System
CTech™ FlowVPX® System delivers in-line, real-time concentration measurement with continuous reading throughout the process.
- Provides accurate measurement during the downstream bioprocess from 0.1 mg/ml to more than 250 mg/ml for an antibody with ε = 1.5 ml/(mg·cm).
- No need to remove samples for testing or disrupt production flow.
- In-line measurements using the FlowVPX system can reduce hold times in production and allow for better process automation.
- Utilized in various stages of the downstream bioprocessing workflow.
ViPER® ANLYTX Software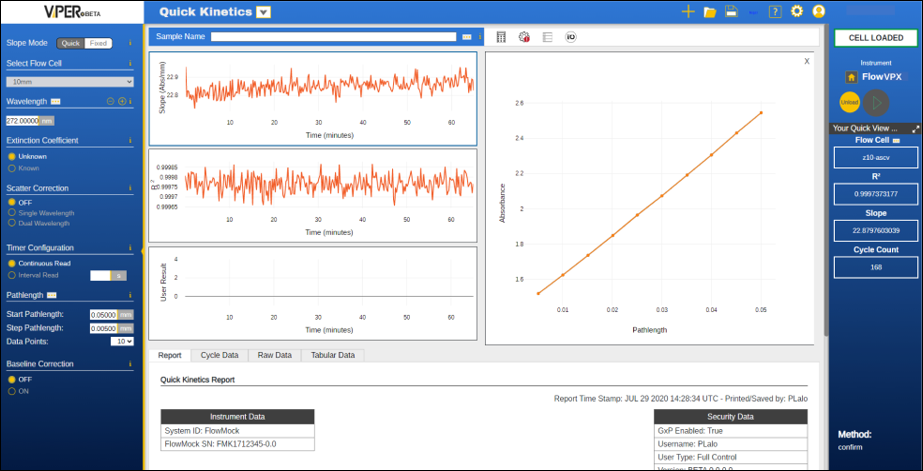
CTech™ ViPER® ANLYTX Software is a next-generation software platform for all your protein concentration measurement (A280) needs. ViPER software features an app-based architecture, permitting easy interpretation of sample data. The software automatically generates key insights from calculations of absorbance over pathlength and displays slope, R2, wavelength, concentration, extinction coefficient (if known), and other user-specified results.
With ViPER software, information enables action.
Protein A280 Industry Data
Using Slope Spectroscopy Methods: Risk Assessment and Cost Savings (Biogen)
 Implementing the SoloVPE System saved Biogen more than 50% in costs ($300,000) and 59 days of labor over a one-year period. This study found that eliminating laboratory inconsistencies such as dilution error, external calculations, limited linear range, sample preparation, and handling makes the SoloVPE System superior platform to traditional fixed pathlength UV technology.
Implementing the SoloVPE System saved Biogen more than 50% in costs ($300,000) and 59 days of labor over a one-year period. This study found that eliminating laboratory inconsistencies such as dilution error, external calculations, limited linear range, sample preparation, and handling makes the SoloVPE System superior platform to traditional fixed pathlength UV technology.
UV-Vis Based Determination of Protein Concentration (Bristol-Myers Squibb)
 Bristol-Myers Squibb validated and implemented slope measurements using variable pathlength technology and compared to traditional UV-vis spectrophotometer methods based on a fixed pathlength (usually 1 cm). Results demonstrated significant efficiency gains using the SoloVPE System with acceptable levels of accuracy, linearity, precision, and robustness.
Bristol-Myers Squibb validated and implemented slope measurements using variable pathlength technology and compared to traditional UV-vis spectrophotometer methods based on a fixed pathlength (usually 1 cm). Results demonstrated significant efficiency gains using the SoloVPE System with acceptable levels of accuracy, linearity, precision, and robustness.
Evaluation of the Slope Spectroscopy Method for Protein Concentration Measurement of Monoclonal Antibodies (Regeneron)
 Regeneron's evaluation of Variable Pathlength Technology (VPT) for their GMP Quality Control Laboratories demonstrated significant benefits. Implementing the SoloVPE System reduced sample prep time, minimized volumes, eliminated background correction, and yielded rapid results. VPT also facilitated easy transferability across labs without extensive analyst training. For labs testing an average of 155 samples weekly, adopting VPT could save approximately nine hours per day or 39 days annually compared to traditional UV-Vis methods.
Regeneron's evaluation of Variable Pathlength Technology (VPT) for their GMP Quality Control Laboratories demonstrated significant benefits. Implementing the SoloVPE System reduced sample prep time, minimized volumes, eliminated background correction, and yielded rapid results. VPT also facilitated easy transferability across labs without extensive analyst training. For labs testing an average of 155 samples weekly, adopting VPT could save approximately nine hours per day or 39 days annually compared to traditional UV-Vis methods.
Application of inline variable pathlength technology for rapid determination of dynamic binding capacity in downstream process development of biopharmaceuticals (KBI Biopharma)
KBI Biopharma used the FlowVPE System to determine dynamic binding capacity of affinity resins to improve resolution of the breakthrough curve—saving at least 2 weeks of analytical testing time. The data show that the resolution of the VPT method is better than the traditional HPLC method. For capture chromatography step breakthrough curve generation, the FlowVPE is an ideal choice to replace the current offline HPLC titer method to realize considerable time and resources savings.
Application of an Effective In-Line Analytical Instrument for Biopharmaceutical Development and Manufacture
 The FlowVPX System’s high-speed continuous data collection, broad concentration-range measurement, and in-line application make it a useful Process Analytics Tool (PAT)—and well suited for effective downstream applications. The broad dynamic pathlength range (0.001–5.000 mm) of VPT removes the need for dilution during measurement by using the system’s search algorithm, which optimizes pathlengths to stay within the linear range of the detector. This unique feature of the FlowVPX system enables in-line application—and thus provides users with means for adapting to the rising demands of modern bioprocessing, such as for analyzing samples with increasingly high expression titers.
The FlowVPX System’s high-speed continuous data collection, broad concentration-range measurement, and in-line application make it a useful Process Analytics Tool (PAT)—and well suited for effective downstream applications. The broad dynamic pathlength range (0.001–5.000 mm) of VPT removes the need for dilution during measurement by using the system’s search algorithm, which optimizes pathlengths to stay within the linear range of the detector. This unique feature of the FlowVPX system enables in-line application—and thus provides users with means for adapting to the rising demands of modern bioprocessing, such as for analyzing samples with increasingly high expression titers.
Expert Webinar Series
The "Go with the Flow" program delves into the transformative power of variable pathlength spectroscopy in enhancing Process Analytical Technology (PAT) for biopharmaceutical development and manufacturing. Join industry as they share insights on implementing in-line PAT solutions, such as CTech FlowVPE/FlowVPX technology, to drive process innovation and efficiency. Discover how these advanced technologies revolutionize protein concentration monitoring, enabling real-time control and automation in biologics production.

Application Services
Your success is our success
Repligen is committed to our customers’ success. We’ve designed our CTech™ Service offerings to supplement our standard support options and provide you with increased access to our highly knowledgeable and experienced professionals. We offer implementation, development, and educational guidance to maximize the benefits of your variable pathlength solutions and the Slope Spectroscopy® technique.
Application Services
- 21 CFR Part 11 Guidance
- Annex 11 Guidance
- Validation Support
- Qualification Service
- Method Transfer Service
Development
- Method Design & Development
- Comparability
- On-Site Support
- Custom Standards
- Process Auditing
Education
- SOP Document Review
- Advanced Slope Spectroscopy Training
- Data Analysis
- Maintenance & Troubleshooting

