OPUS® 2.5 - 80R Pre-packed Chromatography Columns

Discover the OPUS pre-packed chromatography column portfolio, which delivers a comprehensive range of ready-to-use solutions designed to streamline purification processes. Pre-packed columns enable scientists and operators to reduce set up time, ensure consistency and enhance overall process productivity and facility efficiency for biopharmaceutical workflows.
OPUS® 2.5 - 80R cm diameter pre-packed GMP-ready Columns
OPUS® Pre-packed Chromatography Columns provide linear scale-up from process validation to commercial and GMP manufacturing. Select from the broadest portfolio of pre-packed GMP-ready column sizes, including large scale OPUS® 45R, 60R, and 80R Columns. Pre-packed columns enhance facility efficiency by eliminating the need for column packing equipment, space and headcounts so scientists and operators can focus on chromatography runs and accelerate campaign turnaround time.
Innovation in Purification
- Configure bed height to nearest mm
- Pack nearly any resin from nearly any supplier
- Customize plate count, asymmetry and test method
- Unpack resin using recovery port featured on OPUS® 36R, 45R, 60R and 80R columns
- Packed in ISO Class 7 clean rooms
- Complete Regulatory Support File
- Certificate of Analysis for each column configuration
 ULTIMATE FLEXIBILITY
ULTIMATE FLEXIBILITY
Largest scale, broadest range
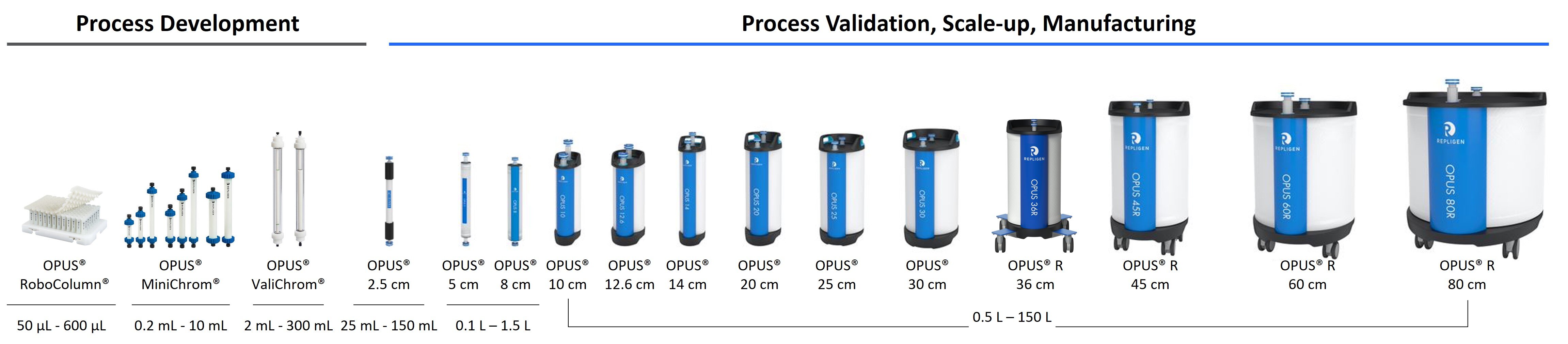
To help scientists easily scale from process development to commercial manufacturing volumes, Repligen offers an extensive assortment of pre-packed chromatography columns which are easily configured to the specifications required by your process. Simply select the resin and bed height, and we will pack, qualify and ship the column to your facility quickly. Additionally, OPUS 36R cm, 45R cm, 60R cm, and 80R cm columns are the first pre-packed chromatography columns designed to meet the requirements of larger 1000L and 2000L single-use bioreactors.
- Easily configured for bed height, resin, and application
- Seamless scale-up from smaller OPUS® Columns
- Complete documentation package for GMP use
- Significant time and cost savings compared to conventional column technology
- Consistent materials of construction
- Class VI, EMA 410/01 compliant materials
- ISO documentation (CoA, RSF)
- Select from > 250 resins
- Individual column release testing

 Ultimate Flexibility
Ultimate Flexibility
Packed with user-specified resins
Repligen packs over 250 commercially available and custom chromatography resins. Typical resins are 30 to 120 um particle size on base bead matrices composed of agarose, methacrylate, cellulose or polystyrene.
Access CHT™ Resin pre-packed in OPUS Columns
Ceramic hydroxyapatite (CHT™) resin offers unique selectivity during the purification of mAbs and recombinant proteins. OPUS Columns can now be pre-packed with CHT™ resin and shipped world wide with excellent performance.
- Internal diameters from 5 - 45 cm
- Bed heights from 5 - 30 cm
View the full performance data
CHT™ is a trademark of Bio-Rad Laboratories, Inc.
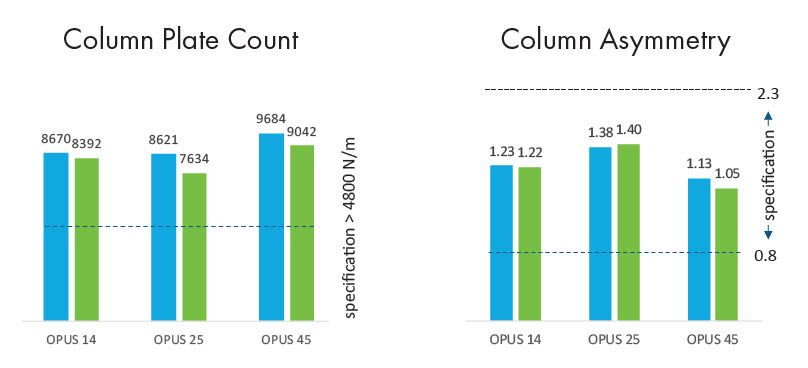
Performance maintained after shipping
Average plate count and asymmetry variance post-shipping was within 8% and 3%, respectively, of the pre-shipping value. Absolute values were within the specification window.
 Ultimate Flexibility
Ultimate Flexibility
Innovative resin recovery port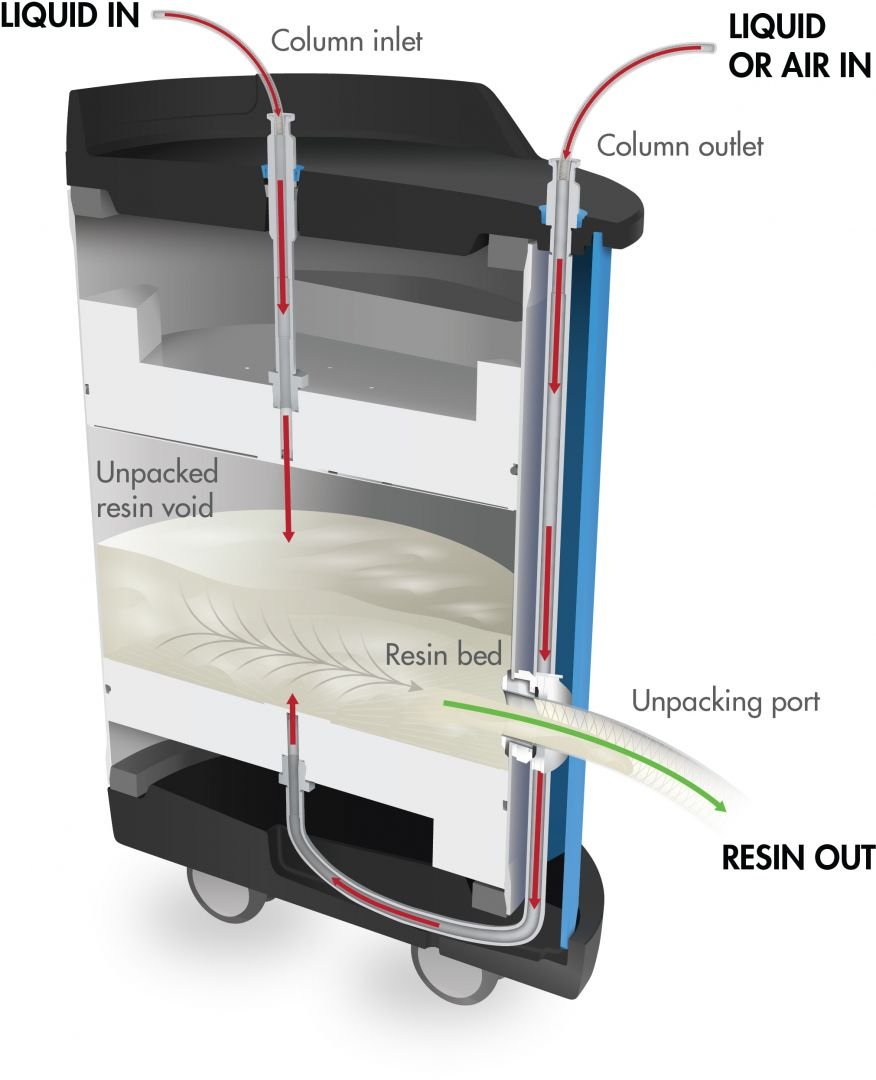
From pre-packed to unpacked
With an innovative side port that allows for easy resin unpacking, OPUS 45R, 60R, and 80R Columns provide the ultimate flexibility in pre-packed column technology. The resin recovery port allows operators to clean and re-use the resin in the OPUS column or a traditional self-packed column.
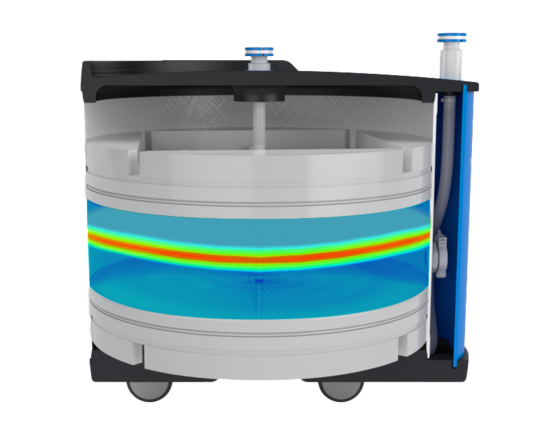
OPUS® flow distributors are designed to maintain uniform flow distribution. The design leverages industry-accepted technology, including a radial flow field and anti-jet funnel.
During the development of OPUS® columns, dispersion as a function of column performance was modeled to confirm critical parameters of the flow distributor. The final design was qualified through computational fluid dynamics modeling and applications case studies performed in the OPUS® applications lab.
Constructed from a medical grade polypropylene homopolymer, all product contact surfaces in OPUS® Columns are EMA 410/01 (or Animal Free) and Class VI Compliant.

OPUS® Columns are compatible with accepted cleaning and sanitization protocols used in typical downstream processing campaigns for monoclonal antibodies, recombinant proteins, or vaccines.
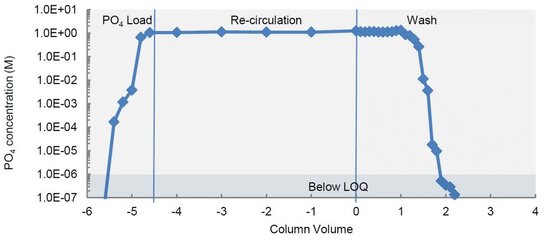
Using colorimetric measurement of phosphate reduction, an OPUS® 20 Column packed with 6 FF was reliably cleaned of residual phosphate in just 2 CVs.
- OPUS® Columns contain no significant dead spaces
- OPUS® Column design is easily cleanable
 HIGH PERFORMANCE
HIGH PERFORMANCE
Impact on process economics
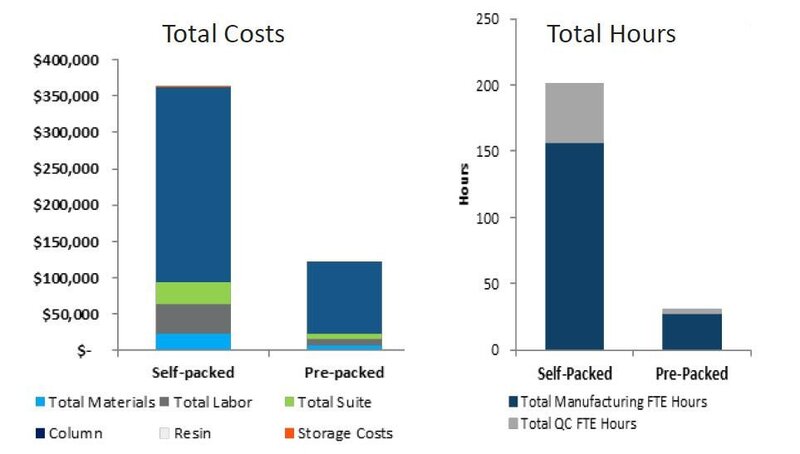
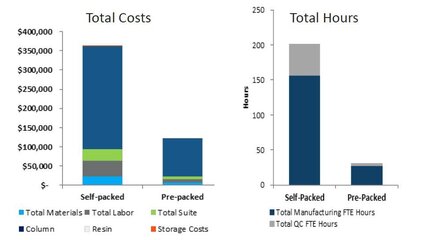
Using pre-packed chromatography columns for clinical and commercial manufacturing processes yields significant cost and labor savings.
- No up-front capital expense
- Reduction of CIP procedures and cross contamination
- Faster campaign turnaround time
- Operational excellence
- Reduced labor requirements
In this example, over 65% cost savings and up to 80% labor savings were achieved when using OPUS Columns compared to self-packed columns.*
*Purchase of OPUS 60R pre-packed column compared to the purchase or a new, 60cm Diameter column and automated self-packing equipment. Data presented is estimated and subject to change.
Click on the + to explore different design features of the OPUS 80R cm column with resin recovery port.

Gamma Irradiation for OPUS 2.5 - 30 cm pre-packed columns
Ensure final product purity and safety using gamma irradiated OPUS columns to reduce bio burden risk. Gamma irradiated columns comply with regulatory standards for product quality and safety and maintain consistent OPUS performance.
- Ideal for large biomolecules where sterile filtration is not an option including: viral vectors, gen therapies and cell therapies
- Risk mitigation to ensure product quality and patient safety
- Plug-and-play solution for continuous downstream processing
- sterility assurance to eliminate bioburden contamination risk
- Facilitated GMP to align to industry best practices
Used resin packing and repacking service
Repligen now offers used resin packing and repacking services. We will repack cleaned and sanitized used resin from used OPUS columns or other pre-packed or self packed columns. Upon receipt of the cleaned and sanitized resin, we will quarantine the resin and column while on site and pack the resin into new OPUS column hard ware or into used OPUS columns (including replacement of ports or other OPUS parts), qualify the column, and return it to our customer. Contact us to learn more about our used resin packing and repacking service.
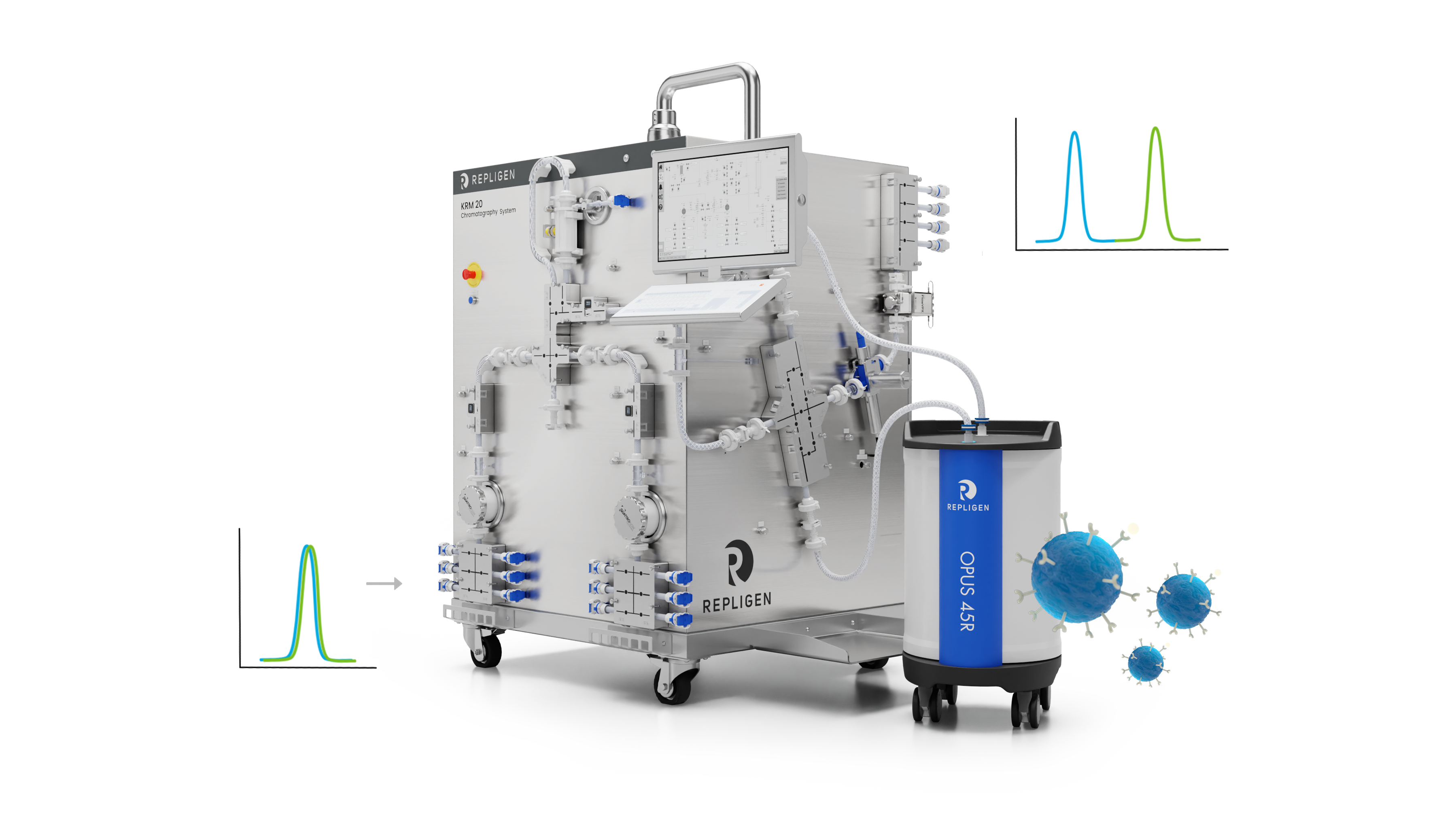
Chromatography Redesigned
Optimize your chromatography workflow with innovative solutions designed to improve product recovery and purity while enhancing operational efficiency. KRM™ chromatography systems, ProConnex® single-use flow paths, AVIPure affinity resins and C-Tech in-line process analytics technology combined with OPUS® pre-packed columns are solutions designed to work together to improve your downstream process productivity.
Click on the image highlights to see key elements of chromatography redesigned.


OPUS® Columns pack your choice of resin and the most precious element of all, time, into a self-packed ready-to-use chromatography column at almost any size and for almost every application. Skip months of hardware validation, execute more runs--and still leave work early to enjoy a bike ride. Don’t worry, we won’t tell your boss.

Reallocate column packing time and resources toward productive GMP-level chromatography, even at small-scale. The family of OPUS® Pre-packed Columns now includes the small but mighty OPUS® 2.5. Scale GMP purification effortlessly from 2.5 cm to 80 cm ID.
Statistical Process Control Analysis
Utilizing statistical process-control analysis (SPA) offers customers valuable insights into process controls and their impact on column performance. This analysis illustrates how variables such as column size, bed height, and diameter affect the process. Years of data from packing columns with hundreds of different resins have been integrated into process-control charting methods and software that leverage data variance and standard deviations to determine control scores. These scores help users to identify which resin types perform optimally under specific conditions.
We continuously evaluate and optimize packing methods through a plan–do–act–check–repeat cycle. For example, we have detailed data from an ion-exchange resin family, showcasing different column diameters and bed heights. Data from a thousand individually packed columns showed efficiency expressed as reduced height equivalent to a theoretical plate (HETP). Over 95% of the data fell within the standard deviation, providing more than 95% confidence in achieving reduced HETP and well-packed columns. Asymmetry trends were similar, with over 97% of results falling within two standard deviations, ensuring consistently packed columns that perform reliably.
Our commitment to quality and precision in column packing is evident through these comprehensive analyses and results.
Manufacturing Centers of Excellence
Repligen develops and manufactures products for the biopharmaceutical industry under an ISO 9001 quality management system. We focus on the timely delivery of high quality, consistent and robust products, to ensure business continuity for our customers.
Repligen manufacturing sites are located in Massachusetts, California, and New Jersey in the United States and in Sweden, France, The Netherlands, Germany and Estonia.


Customer First.
Support is part of the Repligen DNA. Our goal is to provide exceptional customer experience, and to support the efficient and successful adoption and implementation of all Repligen products and services.
- Field Application Support
- Customer Service
- Field Service Engineers